
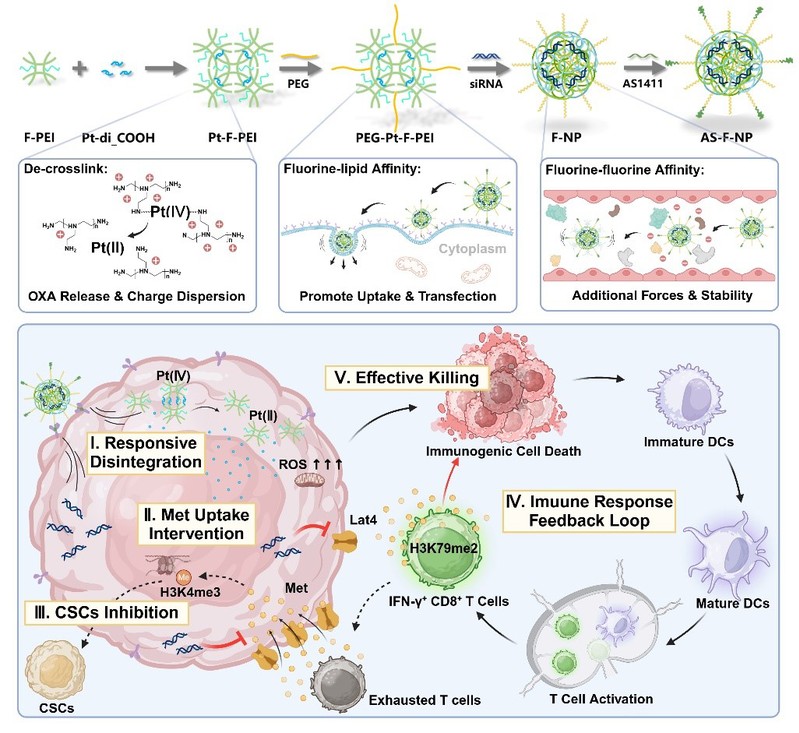
Epigenetic dysregulation in tumors has been established as a pivotal driver of malignant progression, therapeutic resistance, and the formation of a pro-tumor immune microenvironment, mediated through its influence on gene expression and metabolic reprogramming. This uncontrolled metabolic pattern, in turn, ensures the provision of essential resources for tumor proliferation and the plasticity of epigenetic modifications. In recent years, increasing evidence has demonstrated that the greedy nutrient uptake by tumors not only fulfills their own energy and biosynthetic demands but also deprives immune cells of adequate nutrient access. This nutrient-scavenging capability is intrinsically linked to the upregulation of nutrient transporters and metabolic enzymes, driven by dysregulated epigenetic and gene expression mechanisms, thereby forming a tightly interlocked vicious cycle. Consequently, identifying the critical nexus between tumor metabolism and epigenetics may present therapeutic opportunities. Interventions targeting this nexus could simultaneously disrupt the malignant cycle, alleviate chemoresistance, and reverse immune suppression.
In light of this, a co-delivery nanoregulator (AS-F-NP) was designed and constructed by the research team led by Associate Professor Tao Sun and Professor Chen Jiang to intervene in tumor methionine uptake and combine with immunogenic chemotherapy. By silencing Lat4, AS-F-NP limits tumor methionine scavenging, thereby modulating tumor epigenetic plasticity while simultaneously restoring methionine levels in the microenvironment and enhancing the vitality and function of immune effector cells. When combined with immunogenic chemotherapy, AS-F-NP demonstrated remarkable potential in activating anti-tumor immunity and eliminating cancer stem cells in two distinct lung metastasis models. These findings were published online in the internationally renowned journal Advanced Science under the title A Methionine Allocation Nanoregulator for the Suppression of Cancer Stem Cells and Support to the Immune Cells by Epigenetic Regulation.
Boyu Su, the PhD candidate from the School of Pharmacy, Fudan University, is the first author. Associate Professor Tao Sun and Professor Chen Jiang are the corresponding authors of this paper. The work was supported by grants from the National Natural Science Foundation of China, National Key R&D Program of China, Shanghai Municipal Science and Technology Major Project, and ZJLab.
For more information: //advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202415207